Necrotizing fasciitis is a rare but life-threatening bacterial infection that rapidly destroys skin, fat, and the tissue covering muscles (fascia). Often called "flesh-eating disease," it spreads quickly and can lead to severe complications, including organ failure and death, if not treated immediately.
Necrotizing fasciitis is a rare but extremely dangerous bacterial infection that destroys soft tissue at an alarming rate. Often referred to as "flesh-eating disease," it targets the fascia—the connective tissue surrounding muscles, nerves, and blood vessels—causing rapid tissue death (necrosis). Unlike typical infections, this condition spreads aggressively, sometimes within hours, leading to severe complications like sepsis, organ failure, and even death if not treated immediately.
The infection typically enters through breaks in the skin—such as cuts, burns, surgical wounds, or even minor abrasions—allowing bacteria to invade deeper layers. Once inside, the bacteria release toxins that cut off blood supply, killing tissue and creating an environment where the infection thrives. Early symptoms can be deceptive, often resembling less severe conditions like cellulitis, which is why prompt medical attention is critical.
Necrotizing fasciitis is classified into different types based on the bacteria responsible and how they infect the body. Understanding these distinctions helps doctors determine the best treatment approach.
Type I (Polymicrobial Necrotizing Fasciitis) – This is the most common form, accounting for 70-80% of cases. It involves multiple bacteria working together, such as Staphylococcus aureus, Escherichia coli (E. coli), and Klebsiella. Type I often affects people with weakened immune systems, such as diabetics, elderly patients, or those with chronic illnesses. The infection typically starts in the abdomen, groin, or perineal area and spreads rapidly.
Type II (Monomicrobial, Group A Strep Necrotizing Fasciitis) – This type is caused primarily by Group A Streptococcus (GAS), the same bacteria responsible for strep throat. Unlike Type I, Type II can strike otherwise healthy individuals and is more likely to develop after minor injuries, like cuts or bruises. It progresses extremely fast, often leading to toxic shock syndrome.
Type III (Gas Gangrene or Clostridial Necrotizing Fasciitis) – Caused by Clostridium bacteria (commonly C. perfringens), this type is often linked to deep puncture wounds, crush injuries, or surgical complications. A hallmark of this infection is gas production under the skin, detectable by crackling sounds (crepitus) or visible bubbles on imaging. Without treatment, it can be fatal within 24-48 hours.
Type IV (Fungal Necrotizing Fasciitis) – The rarest form, usually caused by fungi like Candida or Mucor, primarily affects immunocompromised patients, such as those with uncontrolled diabetes, cancer, or severe burns. Fungal necrotizing fasciitis is harder to treat and often requires aggressive surgical debridement and antifungal medications.
Each type requires a different medical strategy, but all share one critical factor: time is the enemy. Delayed treatment drastically worsens outcomes, making early recognition and emergency care essential.
Necrotizing fasciitis is notorious for its rapid progression, often worsening within hours. Recognizing the early signs can be the difference between life and death. The symptoms evolve in stages, starting subtly before escalating to severe tissue destruction and systemic illness. Below, we break down the key symptoms in detail.
The initial signs of necrotizing fasciitis can be deceptive, often resembling common skin infections like cellulitis. However, certain red flags distinguish it from less severe conditions:
Severe, Unexplained Pain – One of the earliest and most telling signs is intense pain that seems disproportionate to the visible injury. Patients often describe it as a deep, throbbing ache that worsens rapidly. This happens because the bacteria release toxins that damage nerves and surrounding tissues.
Redness, Swelling, and Warmth – The affected area becomes red, swollen, and hot to the touch. Unlike typical infections, the redness spreads quickly, sometimes at a rate of an inch per hour.
Flu-Like Symptoms – Fever, chills, fatigue, and general malaise develop as the body fights the infection. Nausea, vomiting, and diarrhea may also occur due to toxins entering the bloodstream.
Why It’s Often Missed Early On: Many patients (and even doctors) dismiss these symptoms as a minor infection. However, if pain is extreme and worsening despite antibiotics, necrotizing fasciitis should be suspected.
If untreated, the infection advances rapidly, leading to visible tissue destruction and systemic illness:
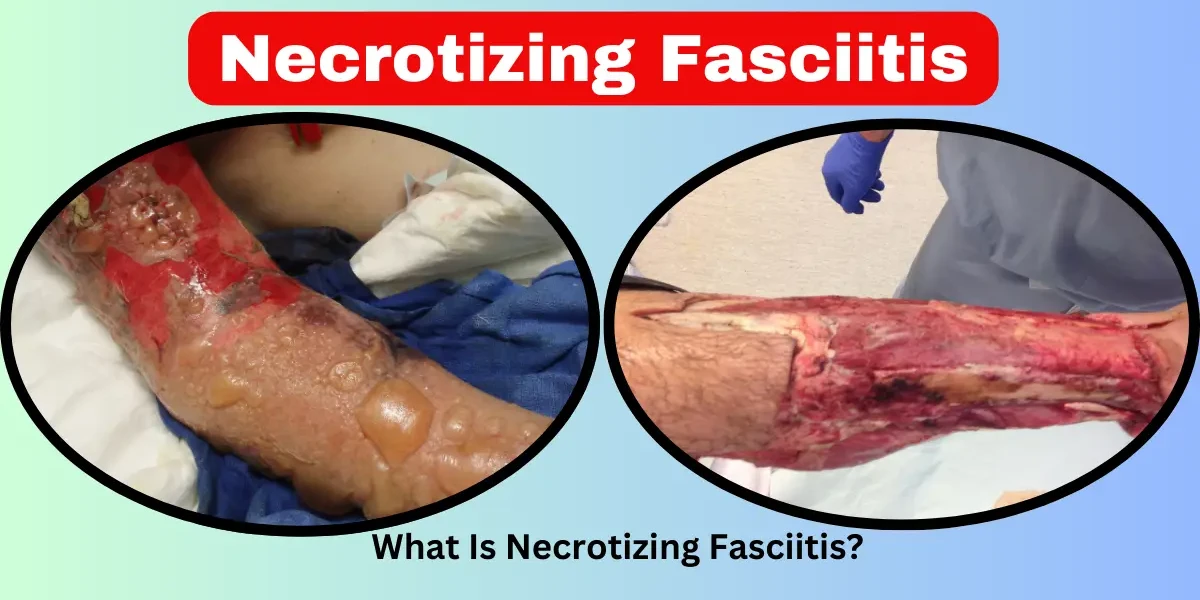
Skin Discoloration (Purple, Black, or Gray Patches) – As blood vessels are destroyed, the skin may turn dark purple, black, or develop a grayish film. This is a clear sign that tissue is dying (necrosis).
Blisters and Bullae – Fluid-filled blisters form, often releasing a foul-smelling, brownish fluid. This is caused by decaying tissue and bacterial byproducts.
Loss of Sensation (Numbness) – As nerves are destroyed, the area may go numb. Ironically, this can temporarily reduce pain, giving a false sense of improvement.
Signs of Sepsis – High fever, rapid heart rate, confusion, and low blood pressure indicate the infection is spreading systemically. This is a medical emergency requiring ICU care.
Case Example: A 2017 study in The New England Journal of Medicine described a patient who developed necrotizing fasciitis after a small scrape. Within 36 hours, their leg turned black, and they went into septic shock. Immediate surgery and IV antibiotics saved their life, but they lost significant muscle tissue.
Without treatment, the infection leads to:
Toxic Shock Syndrome – Bacterial toxins overwhelm the body, causing multi-organ failure.
Gangrene – Complete tissue death requiring amputation.
Unconsciousness and Death – Mortality rates exceed 50% if treatment is delayed beyond 72 hours.
Necrotizing fasciitis occurs when aggressive bacteria invade the body’s soft tissues, releasing toxins that destroy skin, fat, and fascia. While the infection can start from something as minor as a paper cut, certain bacteria and health conditions significantly increase the risk. Understanding these causes and risk factors is crucial for prevention and early intervention.
The most common entry points for bacteria include:
Open wounds (cuts, surgical incisions, puncture wounds)
Burns or abrasions (even small scratches)
Insect bites or animal scratches
Injections or IV drug use (contaminated needles)
Once inside, bacteria multiply rapidly, releasing enzymes and toxins that:
Break down tissue
Cut off blood supply
Trigger widespread inflammation
Different types of bacteria can cause necrotizing fasciitis, including:
Group A Streptococcus (GAS) – The most notorious, linked to severe cases in healthy individuals.
Staphylococcus aureus (including MRSA) – Can cause aggressive infections, especially in hospitals.
Clostridium species – Often found in soil; enters through deep wounds, causing gas gangrene.
Vibrio vulnificus – Found in seawater; affects people with liver disease or weak immune systems.
Aeromonas hydrophila – Present in freshwater; can infect wounds exposed to lakes or rivers.
While anyone can develop necrotizing fasciitis, certain conditions and behaviors significantly increase susceptibility:
Diabetes Mellitus – Poorly controlled diabetes leads to impaired blood circulation (vascular insufficiency) and reduced immune function, making it easier for bacteria to invade and spread. High blood sugar also creates a favorable environment for bacterial growth.
HIV/AIDS – A weakened immune system in HIV patients reduces the body’s ability to fight infections, increasing vulnerability to aggressive bacterial infections.
Chronic Steroid Use or Immunosuppressive Therapy – Patients on long-term corticosteroids, chemotherapy, or immunosuppressants (e.g., organ transplant recipients) have diminished immune responses, making severe infections more likely.
Surgical Wounds or Trauma – Post-surgical infections, especially in contaminated environments, can introduce bacteria deep into tissues.
Injection Drug Use – Needle punctures provide direct entry points for bacteria, particularly when using non-sterile equipment.
Insect Bites, Animal Bites, or Scratches – Even minor skin injuries can become infected if bacteria enter the wound.
Peripheral Vascular Disease (PVD) & Atherosclerosis – Reduced blood flow to extremities impairs wound healing and immune cell delivery, allowing infections to progress unchecked.
Liver Cirrhosis – Impaired liver function reduces the body’s ability to filter toxins and fight infections, increasing susceptibility to severe bacterial infections like Vibrio vulnificus.
Chronic Kidney Disease (CKD) – Uremia and dialysis-related immune dysfunction heighten infection risks.
Alcoholism & Malnutrition – Chronic alcohol abuse weakens immunity and liver function, while malnutrition (particularly protein deficiency) impairs tissue repair.
Obesity – Excess adipose tissue has poor blood supply, creating hypoxic (low-oxygen) conditions where anaerobic bacteria thrive.
Exposure to Contaminated Water – Vibrio vulnificus infections are linked to seawater exposure, while Aeromonas infections occur in freshwater environments.
Elderly Individuals – Aging leads to weakened immunity, slower wound healing, and higher rates of comorbidities like diabetes and vascular disease.
Neonates & Infants – Immature immune systems increase susceptibility to severe bacterial infections.
Necrotizing fasciitis follows a terrifyingly rapid progression that can turn a minor wound into a life-threatening emergency within days - sometimes even hours. Understanding these distinct stages is crucial for early recognition and treatment. Let's examine each phase in detail, including clinical markers and what's happening beneath the skin at each point.
The infection begins subtly but with alarming speed. Bacteria enter through a break in the skin - perhaps something as insignificant as a paper cut, insect bite, or surgical incision. During these first critical hours:
• Bacteria multiply exponentially in the subcutaneous space, secreting toxins that break down tissue
• The body's initial response triggers inflammation, causing the first visible signs
• Patients report severe, disproportionate pain as nerves become irritated by bacterial toxins
• The skin may appear slightly red and swollen, often warmer than surrounding tissue
• Systemic symptoms like low-grade fever (100-101°F), chills, and general malaise emerge
The hidden danger: While surface changes seem mild, beneath the skin, bacteria are already spreading along fascial planes at a rate of about 2-3 cm per hour. This explains why the pain often seems much worse than what's visible.
As we enter the second day, the infection declares itself with unmistakable signs of tissue destruction:
• The skin develops a characteristic dusky, purplish discoloration as blood vessels thrombose
• Bullae (large fluid-filled blisters) form, containing dark, foul-smelling fluid
• Pain transitions to numbness as peripheral nerves are destroyed
• Fever spikes to 103°F or higher as the body mounts a systemic inflammatory response
• Tachycardia (rapid heart rate) and tachypnea (rapid breathing) signal developing sepsis
Pathological changes: At this point, surgical exploration would reveal:
Gray, necrotic fascia that fails to bleed when cut
"Dishwater pus" - a thin, murky fluid with a putrid odor
Fascia that separates easily from surrounding tissue with minimal resistance
By this terminal phase, the infection has overwhelmed local defenses and spread systemically:
• Skin becomes gangrenous - black, leathery, and insensate
• Septic shock develops with plummeting blood pressure (<90 mmHg systolic)
• Multi-organ dysfunction manifests as:
Oliguria (low urine output) from kidney failure
Hypoxia from acute respiratory distress syndrome
Altered mental status from septic encephalopathy
• Laboratory findings show:
Leukocytosis (WBC >20,000) or paradoxically leukopenia
Lactic acidosis (>4 mmol/L)
Coagulopathy with elevated PT/PTT
Mortality rates: Without treatment, mortality approaches:
30% with early, aggressive intervention
70-80% once septic shock develops
Nearly 100% if untreated beyond 96 hours
What makes necrotizing fasciitis so deadly is the narrow window for effective treatment:
Golden period: First 24 hours (optimal outcomes)
Critical period: 24-48 hours (significant morbidity likely)
Irreversible threshold: Beyond 72 hours (high mortality)
This progression explains why the condition carries the grim nickname "flesh-eating disease" - not because bacteria literally eat tissue, but because their toxins create such rapid liquefactive necrosis that tissue seems to melt away before clinicians' eyes.
Diagnosing necrotizing fasciitis quickly is a race against time. Since the infection progresses rapidly—often within hours—doctors rely on a combination of clinical evaluation, lab tests, and imaging to confirm the disease. However, early symptoms can mimic less severe conditions like cellulitis or abscesses, leading to dangerous delays. Here’s a deeper look at the diagnostic process:
The initial diagnosis often begins with a doctor assessing the patient’s symptoms and medical history. Key red flags include:
Severe, disproportionate pain (the pain often seems much worse than the visible injury suggests).
Rapidly spreading redness, swelling, and warmth around a wound.
Fever, chills, nausea, or confusion (signs of systemic infection).
Skin discoloration (purple, black, or gray patches) indicating tissue death.
Doctors may press on the affected area—if the skin feels "crunchy" (crepitus), this suggests gas buildup from bacteria like Clostridium, a hallmark of some necrotizing infections.
Blood work helps confirm an aggressive infection. Key indicators include:
High white blood cell count (leukocytosis) – The body’s response to severe infection.
Elevated C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) – Signs of inflammation.
Low sodium levels (hyponatremia) – A surprising but common finding in necrotizing fasciitis due to bacterial toxins.
Lactate levels – High lactate suggests tissue hypoxia (oxygen deprivation), a sign of worsening infection.
A LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score is sometimes used. A score ≥6 suggests a high likelihood of necrotizing infection, prompting urgent action.
Since necrotizing fasciitis destroys tissue beneath the skin, imaging helps visualize the damage:
X-rays – Can detect gas in tissues (a sign of Clostridium or other gas-producing bacteria).
Ultrasound – May show fluid buildup and thickened fascia.
CT scans – More detailed than X-rays, revealing deeper tissue involvement and gas formation.
MRI – The gold standard for soft tissue imaging, showing fascial thickening and fluid accumulation. However, MRIs take time, which can delay surgery in emergencies.
If necrotizing fasciitis is strongly suspected, surgeons often perform an emergency exploratory procedure. They make a small incision to examine the fascia and muscles. Key findings include:
Gray, necrotic (dead) tissue that doesn’t bleed.
Foul-smelling discharge (a sign of bacterial decay).
"Dishwater pus" – A thin, murky fluid instead of typical thick pus.
A tissue biopsy may be sent to the lab for immediate analysis to confirm bacterial presence and guide antibiotic selection.
Early symptoms resemble common infections (cellulitis, abscesses), leading to misdiagnosis.
Pain medications can mask worsening symptoms, delaying intervention.
Not all cases show classic signs (like gas on imaging), especially in early stages.
Case Example: A 2017 study in The American Journal of Emergency Medicine found that nearly 30% of necrotizing fasciitis cases were initially misdiagnosed, leading to worse outcomes.
Treating necrotizing fasciitis is a race against time. Since the infection spreads rapidly—sometimes at a rate of one inch per hour—immediate medical intervention is crucial. The primary treatment approach combines emergency surgery, powerful antibiotics, and intensive supportive care. Here’s a deeper look at each component:
The most critical step in treating necrotizing fasciitis is surgical debridement, where surgeons remove all infected and dead tissue to stop the infection from spreading. This procedure is often performed within hours of diagnosis because delays drastically reduce survival rates.
How It Works: Surgeons cut away blackened, dying tissue until only healthy, bleeding tissue remains.
Multiple Surgeries May Be Needed: Since bacteria can continue spreading, patients often require repeated operations over days or weeks.
Amputation in Severe Cases: If the infection reaches a limb and cannot be controlled, doctors may amputate to save the patient’s life.
Real-Life Example: A 2016 case study in The New England Journal of Medicine described a patient who underwent 12 surgeries in two weeks to fully remove infected tissue. Without such aggressive treatment, the outcome would have been fatal.
While surgery removes infected tissue, high-dose IV antibiotics are essential to kill bacteria in the bloodstream and remaining tissues. Doctors typically start with broad-spectrum antibiotics (effective against multiple bacteria) and later adjust based on lab results.
First-Line Antibiotics:
Penicillin + Clindamycin – Effective against Group A Streptococcus (common in Type II infections).
Carbapenems or Piperacillin-Tazobactam – Used for polymicrobial (Type I) infections.
Vancomycin or Daptomycin – Added if antibiotic-resistant Staphylococcus (MRSA) is suspected.
Why Clindamycin Is Critical: Unlike penicillin, clindamycin blocks bacterial toxin production, which helps slow tissue destruction.
Did You Know? Some hospitals use antibiotic combinations within 30 minutes of suspected diagnosis to maximize effectiveness.
Because necrotizing fasciitis can cause sepsis, organ failure, and shock, patients need intensive monitoring and life-supporting treatments, including:
IV Fluids & Vasopressors – To maintain blood pressure and prevent septic shock.
Pain Management – High-dose opioids or nerve blocks due to extreme pain from tissue damage.
Hyperbaric Oxygen Therapy (HBOT) – Some hospitals use oxygen chambers to increase oxygen delivery to damaged tissues, slowing bacterial growth. (However, this is controversial and not always available.)
Blood Transfusions & Dialysis – If kidneys fail due to toxins.
Even after surviving the infection, patients often face:
Skin grafts or reconstructive surgery to repair lost tissue.
Physical therapy to regain mobility if muscles were affected.
Psychological support for trauma, PTSD, or depression after a near-death experience.
While necrotizing fasciitis is rare, its devastating effects make prevention crucial. Since the infection often enters through breaks in the skin, proper wound care and hygiene are the best defenses. Here’s how you can reduce your risk:
Any cut, scrape, or puncture wound—no matter how small—should be cleaned right away with soap and clean running water. This removes dirt and bacteria that could lead to infection. For deeper wounds, applying an antiseptic solution (like hydrogen peroxide or iodine) can further reduce bacterial growth. Avoid using harsh substances like alcohol directly on open wounds, as they can damage tissue and delay healing.
After cleaning, cover the wound with a sterile bandage or dressing to prevent bacteria from entering. Change the bandage daily or whenever it becomes wet or dirty. If you’re in an environment where exposure to dirt or bacteria is likely (such as while gardening, swimming, or working with animals), use waterproof bandages or protective gloves. Open wounds should never be left exposed, especially in high-risk settings like hospitals or contaminated water sources.
Even with proper care, infections can develop. Watch for increasing pain, redness, swelling, warmth, or pus around a wound. If these symptoms appear—especially if accompanied by fever or chills—seek medical attention immediately. Early antibiotic treatment can stop a simple infection from turning into necrotizing fasciitis.
People with diabetes, weakened immune systems, or poor circulation are at higher risk. If you have diabetes, keep blood sugar levels under control to improve healing and reduce infection risks. Those with autoimmune diseases or undergoing treatments like chemotherapy should take extra precautions, including regular skin checks and prompt medical care for any injuries.
Necrotizing fasciitis can enter through wounds exposed to seawater, brackish water, or even hot tubs with improper chlorine levels. If you have cuts or open sores, avoid swimming in natural bodies of water or public pools until fully healed.
Since bacteria like Streptococcus and Staphylococcus can spread through contact, frequent handwashing with soap is essential—especially before touching wounds. Avoid sharing personal items like razors, towels, or needles, as these can transfer harmful bacteria.
Deep wounds, animal bites, or injuries contaminated with dirt or saliva require professional medical evaluation. Doctors may prescribe preventive antibiotics or recommend a tetanus shot if needed.
While necrotizing fasciitis can’t always be prevented, these steps significantly lower the risk. Being proactive about wound care and infection signs could mean the difference between a minor injury and a life-threatening condition. If in doubt, always err on the side of caution and consult a healthcare provider.
Even with prompt and aggressive treatment, necrotizing fasciitis can leave survivors with severe and sometimes lifelong complications. The infection’s rapid destruction of tissue, combined with the body’s extreme inflammatory response, often leads to lasting damage. Below, we explore the major complications in detail.
One of the most visible complications of necrotizing fasciitis is extensive scarring and disfigurement. Because the infection destroys large areas of skin, fat, and muscle, surgical removal of dead tissue (debridement) often leaves deep wounds. Skin grafts or reconstructive surgeries may be necessary, but even with advanced medical care, significant scarring remains. In some cases, patients require multiple plastic surgeries over years to restore function and appearance. The psychological impact of these physical changes can be profound, affecting self-esteem and social interactions.
When necrotizing fasciitis spreads to limbs, amputation may be the only way to save a patient’s life. The infection can cut off blood supply, destroy nerves, and cause irreversible tissue death, making salvage impossible. Losing a limb leads to immediate physical challenges, including learning to use prosthetics or wheelchairs. Rehabilitation is often long and painful, requiring extensive physical therapy to regain mobility. For some survivors, permanent disability affects their ability to work or perform daily activities independently.
Necrotizing fasciitis doesn’t just attack the skin—it can also lead to systemic complications like sepsis (a life-threatening body-wide infection) and toxic shock syndrome. These conditions can cause lasting damage to vital organs, including the kidneys, liver, heart, and lungs. Survivors may develop chronic kidney disease, heart failure, or lung scarring (pulmonary fibrosis). Some patients require long-term dialysis or other medical support due to organ dysfunction. Additionally, the body’s extreme immune response can trigger autoimmune-like conditions, leading to ongoing inflammation and pain.
The infection and subsequent surgeries can damage nerves, leading to chronic pain syndromes like complex regional pain syndrome (CRPS) or neuropathy. Some survivors experience persistent burning, tingling, or hypersensitivity in affected areas. Even after healing, scar tissue can press on nerves, causing ongoing discomfort. Pain management often becomes a long-term challenge, requiring medications, nerve blocks, or alternative therapies like acupuncture.
Surviving necrotizing fasciitis can be traumatic, leaving deep emotional scars. Many patients develop post-traumatic stress disorder (PTSD), depression, or anxiety due to the sudden, life-threatening nature of the illness and the painful treatments involved. The drastic changes in physical appearance and abilities can also lead to body image issues and social withdrawal. Counseling, support groups, and mental health care are often crucial for recovery.
Necrotizing fasciitis is a medical nightmare, but awareness saves lives. Recognizing Necrotizing Fasciitis Symptoms and Signs early, understanding Necrotizing Fasciitis Causes and Risk Factors, and seeking immediate treatment are crucial.
If you or someone shows sudden, severe pain with skin changes—don’t wait. Go to the ER. With rapid Necrotizing Fasciitis Diagnosis and Treatment, survival and recovery are possible.
Necrotizing fasciitis, commonly known as "flesh-eating disease," is a severe bacterial infection that rapidly destroys skin, fat, and the tissue covering the muscles (fascia). The primary causes are bacterial infections, most commonly Group A Streptococcus (Streptococcus pyogenes), but other bacteria like Staphylococcus aureus, Clostridium, Vibrio vulnificus (from seawater), and polymicrobial infections can also be responsible. The bacteria enter the body through breaks in the skin, such as cuts, burns, surgical wounds, or insect bites. People with weakened immune systems, diabetes, or chronic illnesses are at higher risk. The infection spreads quickly, releasing toxins that kill tissue and compromise blood flow, leading to widespread necrosis.
Treatment for necrotizing fasciitis must be immediate and aggressive to prevent fatal complications. The primary treatment is surgical debridement, where doctors remove all infected and dead tissue to stop the spread. In severe cases, amputation may be necessary. Intravenous broad-spectrum antibiotics (e.g., penicillin, clindamycin, and vancomycin) are administered to combat the infection. Supportive care includes IV fluids, pain management, and sometimes hyperbaric oxygen therapy to improve oxygen delivery to tissues. Patients often require intensive care due to the risk of sepsis, organ failure, or toxic shock syndrome.
Skin necrosis (tissue death) presents with several key symptoms:
Discoloration: The skin may turn purple, black, or dark red due to lack of blood flow.
Severe pain that worsens rapidly, often disproportionate to visible symptoms.
Swelling, warmth, and redness around the infected area.
Blisters or ulcers filled with foul-smelling fluid or pus.
Fever, chills, fatigue, and dizziness as the infection spreads systemically.
Numbness in later stages as nerves are destroyed.
Early recognition is crucial, as delays can lead to life-threatening complications.
The survival rate depends on how quickly treatment is initiated. With early diagnosis and aggressive surgery, survival rates range from 70-90%. However, if treatment is delayed, mortality can exceed 30-50%, especially in cases involving toxic shock or organ failure. Factors like the patient’s overall health, the bacteria involved (e.g., Vibrio vulnificus has a higher fatality rate), and the infection’s extent also influence outcomes. Survivors often require long-term rehabilitation and may suffer from scarring, limb loss, or permanent disability.
Necrotizing fasciitis is not highly contagious, but the bacteria causing it (e.g., Group A Strep) can spread through direct contact with open wounds or bodily fluids. However, most people exposed to these bacteria do not develop necrotizing fasciitis unless they have open wounds or weakened immunity. Proper hygiene, wound care, and avoiding contact with infected wounds reduce transmission risk. In rare cases, outbreaks have occurred in healthcare settings.
Yes, necrotizing fasciitis can be cured if treated early and aggressively. The key is immediate surgical intervention to remove dead tissue and strong IV antibiotics to kill the bacteria. However, delayed treatment increases the risk of death or severe complications like limb amputation or organ failure. Survivors may need skin grafts, physical therapy, and psychological support due to the trauma of the disease.
Type 2 necrotizing fasciitis is caused by Group A Streptococcus (GAS), sometimes with Staphylococcus aureus (including MRSA). It is monomicrobial (one main bacteria) and tends to progress very rapidly, often leading to toxic shock syndrome. It commonly affects healthy individuals after minor injuries. Type 1, in contrast, is polymicrobial (multiple bacteria) and usually occurs in people with diabetes or vascular disease.
Recovery varies based on severity but often takes weeks to months. After surgery, patients may stay in the hospital for weeks for wound care and antibiotics. Skin grafts or reconstructive surgery may be needed. Physical therapy helps restore mobility, especially if amputation occurred. Emotional recovery is also important, as survivors may experience PTSD or depression from the traumatic experience.
Treatment depends on severity:
Minor necrosis: Wound cleaning, antibiotic ointments, and dressings.
Moderate cases: Debridement (removal of dead tissue) by a doctor.
Severe cases: Surgical excision, skin grafts, or hyperbaric oxygen therapy.
Underlying causes (e.g., diabetes, infections) must also be managed.
Never ignore skin necrosis, as it can worsen rapidly.
Small amounts of necrotic tissue (e.g., in diabetic ulcers) can sometimes be managed at home with:
Sterile saline washes to clean the wound.
Hydrogel or medicated dressings to promote healing.
Gentle debridement with a sterile tool (only if advised by a doctor).
However, deep or extensive necrosis requires medical intervention—attempting self-removal can cause severe infection or bleeding.
Early-stage necrosis can be extremely painful due to inflammation and nerve irritation. However, as tissue dies, nerves are destroyed, leading to loss of sensation in the affected area. Pain elsewhere may persist due to infection spread or systemic illness (e.g., sepsis). Pain management is crucial in treatment.
Do not squeeze or puncture pus-filled wounds, as this can push bacteria deeper. Instead:
Clean with mild soap and water.
Apply a warm compress to encourage natural drainage.
Cover with a sterile bandage.
See a doctor if there’s increased pain, redness, or fever, as this may indicate an abscess needing drainage or antibiotics.
You Might Also Like